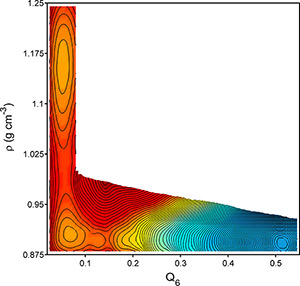
Princeton University researchers conducted computer simulations to explore what happens to water as it is cooled to temperatures below freezing and found that the supercooled liquid separated into two liquids with different densities. The finding agrees with a two-decade-old hypothesis to explain water’s peculiar behaviors, such as becoming more compressible and less dense as it is cooled. The X axis above indicates the range of crystallinity (Q6) from liquid water (less than 0.1) to ice (greater than 0.5) plotted against density (ρ) on the Y axis. The figure is a two-dimensional projection of water’s calculated “free energy surface,” a measure of the relative stability of different phases, with orange indicating high free energy and blue indicating low free energy. The two large circles in the orange region reveal a high-density liquid at 1.15 g/cm3 and low-density liquid at 0.90 g/cm3. The blue area represents cubic ice, which in this model forms at a density of about 0.88 g/cm3. (Image courtesy of Jeremy Palmer)
